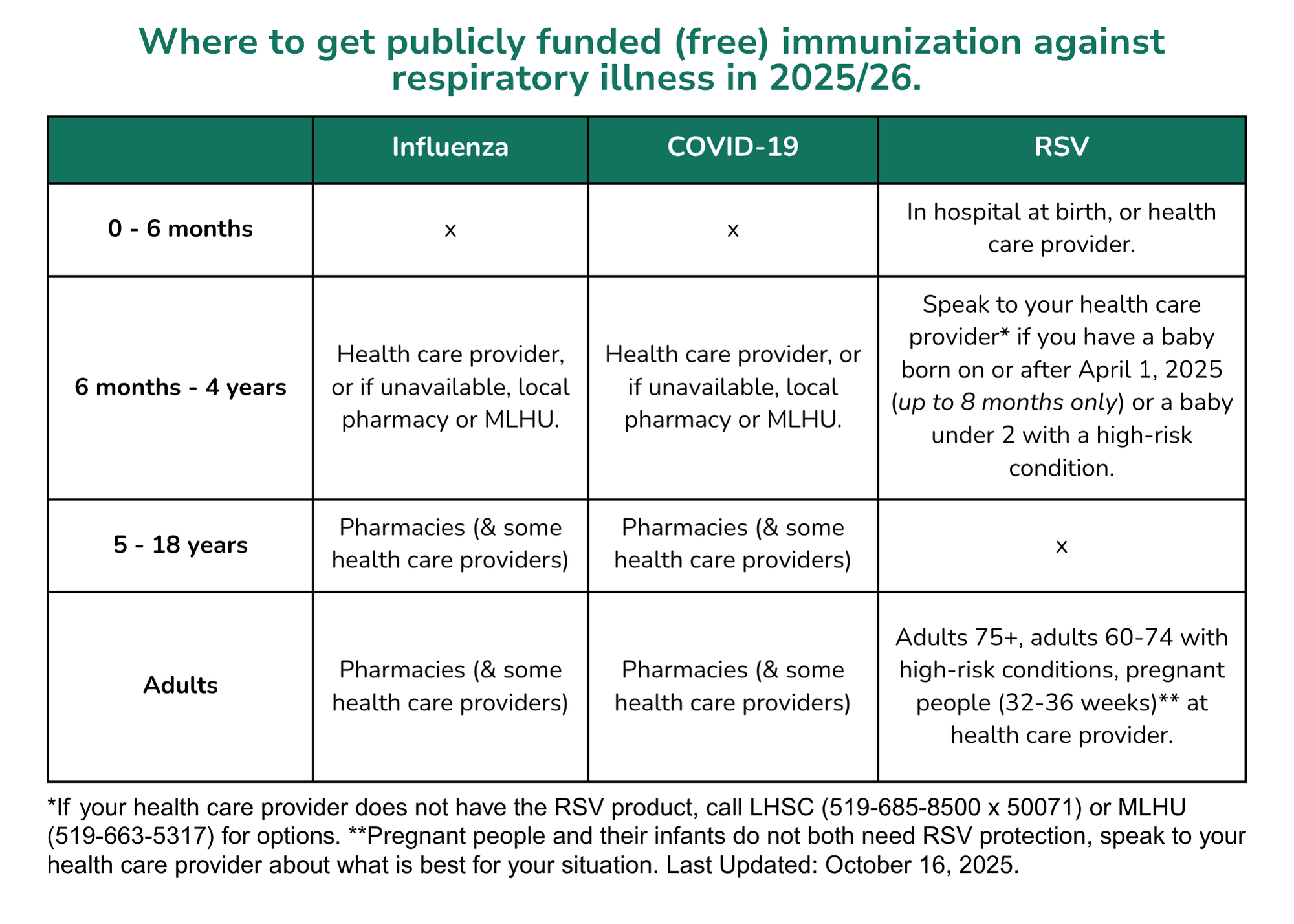
COVID-19 Vaccine
Getting vaccinated and staying up to date with your COVID-19 vaccine doses remains the most effective way to reduce severity of symptoms and to prevent serious illness (including hospitalization and death) from COVID-19 infection.
Ontario’s COVID-19 program has transitioned to an annual program. In the fall of 2025, all individuals 6 months of age and older who live, work, or study in Ontario are eligible to receive one dose of COVID-19 vaccine.
Some individuals may also be indicated to have a second dose this fall (children 6 months to 4 years of age and those with certain conditions), while other specific populations will be eligible to receive a subsequent vaccine dose in the spring of 2026.
COVID-19 Vaccines - Where to Access
Eligible individuals in Ontario will be able to receive vaccine at a participating pharmacy or at their health care provider in the fall of 2025. Call first to see if vaccine is available and if an appointment is required. COVID-19 vaccine is available free of charge for everyone aged 6 months of age and older who lives, works or goes to school in Ontario, and is available without a health card.
For families with children four years of age and younger, please call the Middlesex-London Health Unit (519-663-5317) for options if there are difficulties obtaining a dose.
COVID-19 Vaccines - When to Get Your Next Dose
Everyone aged 6 months of age and older who lives, works or goes to school in Ontario may receive their COVID-19 vaccine dose(s) starting on October 27, 2025.
Individuals may be indicated to receive a dose earlier than October 27,2025. These specific populations are outlined on the Ministry of Health’s COVID-19 website.
Individuals should receive a vaccine dose as soon as they are eligible. Spacing from last dose of COVID-19 vaccine should be at least 3 months (84 days). If you have had COVID-19 illness within the last 3 months or are immunocompromised, please speak with your health care provider. More information is also available here.
COVID-19 vaccines can be given at the same time, same day or before or after any other vaccine dose (e.g., flu vaccines, routine vaccines).
COVID-19 Vaccines - Products and Safety
For the Fall 2025season, all vaccine products have been updated to contain protection against the LP.8.1 variant.
Vaccines have been approved by Health Canada. All products have gone through rigorous quality control and assurance testing and have been deemed as safe and effective.
All COVID-19 vaccines available this year are mRNA vaccines. This means they use mRNA to teach the body’s cells how to make a protein which will cause your body to have an immune response and make antibodies. These antibodies then help fight an infection if the virus, which causes COVID-19, later enters the body. These vaccines do not contain a live virus – they cannot give you COVID-19 illness.
NOTE: Novavax vaccine (non-mRNA) is not available in Ontario for the 2025/2026 season
Children and Youth
When eligible for a dose during respiratory season, children can begin receiving COVID-19 vaccine starting at 6 months old. The vaccines are safe, effective and are the best way to remain protected your child and those closest to them from COVID-19 and its variants.
There is a lot of information available for families to consider when making decisions about the COVID-19 vaccine for children. Please visit:
- www.ontario.ca/page/covid-19-vaccines
- www.canada.ca/en/public-health/services/vaccination-children/covid-19.html
- Visit VaxFacts+ for information about booking a one-to-one phone consultation
Parents, legal guardians and care givers can also speak to the child's health care provider or pharmacist. Public Health Nurses at the Middlesex-London Health Unit can also provide information (call 519-663-5317).
Side Effects
As with most vaccines, common side effects have been reported. These side effects are likely to be moderate and resolve after a few days. In very rare situations, some people may experience serious symptoms or an adverse reaction. For more information, visit Health Canada. Report all serious side effects to your healthcare provider.
When should I speak with a healthcare provider prior to immunization?
Contact your healthcare provider if you have questions about getting the vaccine, or if the individual:
- is immunocompromised due to disease or treatment (to discuss optimal timing for immunization)
- has had a severe allergic reaction following a previous COVID-19 vaccine, or has an allergy to a component of the vaccine
- was diagnosed with myocarditis or pericarditis (inflammation in different parts of the heart) following immunization with an mRNA vaccine
COVID-19 Vaccine Certificate
To access your COVID-19 vaccine certificate, please visit: covid-19.ontario.ca/get-proof. NOTE: you will need your date of birth, postal code and health card information.
If you don't have a green Ontario Health Card or used another form of identification (e.g. Driver’s License) at the time of vaccination, or if you have questions or concerns about your vaccine certificate, please contact the Provincial Vaccine Contact Centre.
Guidance for Individuals Vaccinated Outside of Ontario/Canada
Residents of Ontario who received COVID-19 vaccine(s) outside of Ontario/Canada, should keep any documentation or records of these personal files. These out of province records may be accepted by employers, educational institutions, health care providers and pharmacists as proof of vaccination.
Who can report
Reporting vaccines that were administered outside of the province or country will not be included in Ontario's provincial COVID-19 vaccine database (COVAXon), except in specific situations, to ensure future doses can be given safely and at the appropriate intervals. Individuals meeting the following criteria should report their out-of-Ontario/Canada records:
- children younger than 5 years
- individuals aged 5 years and older who received 1 or more doses outside of Ontario/Canada within the last 6 months
Other records will not be added to the provincial database at this time.
How to report
Individuals who meet the above criteria can report their out-of-Ontario/Canada COVID-19 vaccine doses to be included in the provincial data base through the Middlesex-London Health Unit.
- Online reporting
- May take up to 14 days to process
- An Ontario Health Card is not required. If you do not have an Ontario Health Card, you will be prompted to upload another piece of ID (birth certificate, driver's license, employee ID, Indian Status Card or Indigenous Membership Card, passport, or out of province health card).
- A clear photo of the entire record is required.
- If you are unable to submit proof electronically, please call 519-663-5317 for further direction.
How to access your Ontario proof of vaccination
Once out-of-Ontario/Canada COVID-19 vaccine doses are reported and the submission has been processed, individuals with a valid Ontario Health Card can download their proof of vaccination online at Proof of COVID-19 Vaccination. Individuals without an Ontario Health Card can obtain a client ID and link to download their proof of vaccination by calling 519-663-5317 (Monday to Friday between 8:30AM and 4:30PM).
Last modified on: October 16, 2025
References
https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci.html
https://www.ontario.ca/page/covid-19-vaccines
https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines.html
https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/signs-symptoms-severity.html#a3
https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/type-mrna.html
https://covid-19.ontario.ca/proof-covid-19-vaccination
https://www.ontario.ca/page/covid-19-vaccine-program